Smith Family- Cosmetic Dentistry 1112 Nc H Ighway 120 Sneads Ferry N

The Safety of COVID-19 Vaccinations—Nosotros Should Rethink the Policy
1
Poznan University of the Medical Sciences, Pediatric Hospital, 60-572 Poznan, Poland
two
Department of Psychology, University of Witten/Herdecke, 58448 Witten, Deutschland
3
Change Wellness Science Institute, 10178 Berlin, Deutschland
4
Section of Radiation Oncology, Leopoldina Hospital, 97422 Schweinfurt, Germany
5
Independent Data and Pattern Scientist, Brinkenbergweg 1, 7351 BD Hoenderloo, The Netherlands
*
Author to whom correspondence should be addressed.
Bookish Editor: Ralph J. DiClemente
Received: two June 2021 / Revised: 19 June 2021 / Accepted: 21 June 2021 / Published: 24 June 2021
Abstract
Background: COVID-nineteen vaccines accept had expedited reviews without sufficient safety data. Nosotros wanted to compare risks and benefits. Method: Nosotros calculated the number needed to vaccinate (NNTV) from a large Israeli field study to prevent i expiry. We accessed the Adverse Drug Reactions (ADR) database of the European Medicines Agency and of the Dutch National Register (lareb.nl) to extract the number of cases reporting astringent side effects and the number of cases with fatal side effects. Result: The NNTV is between 200–700 to prevent one case of COVID-19 for the mRNA vaccine marketed by Pfizer, while the NNTV to forbid 1 death is betwixt 9000 and fifty,000 (95% confidence interval), with sixteen,000 as a betoken judge. The number of cases experiencing adverse reactions has been reported to be 700 per 100,000 vaccinations. Currently, we see 16 serious side effects per 100,000 vaccinations, and the number of fatal side effects is at four.11/100,000 vaccinations. For iii deaths prevented past vaccination we have to accept two inflicted by vaccination. Conclusions: This lack of articulate do good should cause governments to rethink their vaccination policy.
1. Introduction
In the form of the SARS-CoV2 pandemic, new regulatory frameworks were put in place that allowed for the expedited review of data and access of new vaccines without safety data [1]. Many of the new vaccines use completely new technologies that accept never been used in humans before. The rationale for this activeness was that the pandemic was such a ubiquitous and dangerous threat that it warrants infrequent measures. In due course, the vaccination campaign against SARS-CoV2 has started. To date (18 June 2021), roughly 304.5 meg vaccination doses have been administered in the Eu (https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#distribution-tab (accessed on 18 June 2021)), mostly the vector vaccination product adult past the Oxford vaccination group and marketed by AstraZeneca, Vaxzevria [2] (approximately 25% coverage in the EU), the RNA vaccination product of BioNTec marketed past Pfizer, Comirnaty [3,4] (approximately 60%), and the mRNA vaccination production developed by Moderna [5] (approximately 10%). Others business relationship for only around 5% of all vaccinations. As these vaccines have never been tested for their safety in prospective postal service-marketing surveillance studies, we thought it useful to determine the effectiveness of the vaccines and to compare them with the costs in terms of side effects.
2. Methods
We used a large Israeli field study [six] that involved approximately one 1000000 persons and the data reported therein to summate the number needed to vaccinate (NNTV) to prevent one case of SARS-CoV2 infection and to prevent one death caused by COVID-nineteen. In addition, we used the most prominent trial data from regulatory phase 3 trials to assess the NNTV [4,v,7]. The NNTV is the reciprocal of the absolute take chances difference between risk in the treated group and in the command group, expressed as decimals. To give an artificial example: An absolute chance difference between a risk of 0.eight in the control grouping and a risk of 0.3 in the treated group would result in an accented risk deviation of 0.5; thus, the number needed to care for or the NNTV would exist 1/0.5 = 2. This is the clinical effectiveness of the vaccine.
We checked the Adverse Drug Reaction (ADR) database of the European Medicine Agency (EMA: http://www.adrreports.eu/en/search_subst.html#, accessed on 28 May 2021; the COVID-xix vaccines are accessible under "C" in the alphabetize). Looking up the number of single cases with side effects reported for the iii most widely used vaccines (Comirnaty by BioNTech/Pfizer, the vector vaccination product Vaxzevria marketed by AstraZeneca, and the mRNA vaccine by Moderna) past country, nosotros discovered that the reporting of side effects varies by a cistron of 47 (Figure one). While the European average is 127 individual case safety reports (ICSRs), i.e., cases with side upshot reports, per 100,000 vaccinations, the Dutch authorities have registered 701 reports per 100,000 vaccinations, while Poland has registered only 15 ISCRs per 100,000 vaccinations. Assuming that this deviation is not due to differential national susceptibility to vaccination side furnishings, but due to dissimilar national reporting standards, nosotros decided to utilise the data of the Dutch national register (https://world wide web.lareb.nl/coronameldingen; accessed on 29 May 2021) to gauge the number of astringent and fatal side effects per 100,000 vaccinations. We compare these quantities to the NNTV to prevent 1 clinical instance of and one fatality by COVID-19.
3. Results
Cunningham was the get-go to indicate out the loftier NNTV in a non-peer-reviewed annotate: Around 256 persons needed to vaccinate with the Pfizer vaccine to forbid 1 example [viii]. A recent large field study in Israel with more than a one thousand thousand participants [6], where Comirnaty, the mRNA vaccination product marketed past Pfizer, was applied allowed the states to summate the figure more precisely. Table 1 presents the data of this study based on matched pairs, using propensity score matching with a large number of baseline variables, in which both the vaccinated and unvaccinated persons were even so at run a risk at the beginning of a specified catamenia [vi]. We mainly used the estimates from Table 1, considering they are likely closer to existent life and derived from the largest field report to date. However, we also report the information from the stage 3 trials conducted for obtaining regulatory approval in Table 2 and used them for a sensitivity analysis.
It should be noted that in the Israeli field written report, the cumulative incidence of the infection, visible in the control grouping afterwards 7 days, was low (Kaplan–Meier guess <0.5%; Effigy 2 in Dagan et al.'s piece of work [6]) and remained below 3% afterwards half-dozen weeks. In the other studies, the incidence figures afterwards three to six weeks in the placebo groups were similarly depression, betwixt 0.85% and ane.8%. The absolute infection gamble reductions given by Dagan et al. [6] translated into an NNTV of 486 (95% CI, 417–589) two to three weeks afterward the first dose, or 117 (90–161) subsequently the second dose until the end of follow-up to prevent one documented instance (Tabular array i). Estimates of NNTV to prevent CoV2 infection from the phase iii trials of the nigh widely used vaccination products [3,4,5] were between 61 (Moderna) and 123 (Tabular array 2) and were estimated to be 256 past Cunningham [eight]. Nonetheless, information technology should as well be noted that the outcome "Documented infection" in Table 1 refers to CoV2 infection as defined by a positive PCR examination, i.e., without considering simulated-positive results [x], so that the outcome "symptomatic affliction" may improve reflect vaccine effectiveness. If clinically symptomatic COVID-nineteen until the end of follow-up was used as an event, the NNTV was estimated as 217 (95% CI, 154–304).
In the Israeli field study, 4460 persons in the vaccination group became infected during the study period and ix persons died, translating into an infection fatality charge per unit (IFR) of 0.2% in the vaccination grouping. In the control group, 6100 became infected and 32 died, resulting in an IFR of 0.5%, which is within the range institute by a review [xi].
Using the data from Table one, nosotros calculated the absolute risk deviation to be 0.00006 (ARD for preventing one death after three to iv weeks), which translates into an NNTV of sixteen,667. The 95% conviction interval spanned the range from 9000 to 50,000. Thus, betwixt 9000 and 50,000 people need to be vaccinated, with a point-estimate of roughly 16,000, to forbid 1 COVID-19-related decease.
For the other studies listed in Table 2, in the case that positive infection was the outcome [7], we calculated the NNTV to prevent one death using the IFR approximate of 0.5%; in the instance that clinically positive COVID-19 was the outcome [4,five], we used the case fatality rate estimated as the number of worldwide COVID-19 cases divided by COVID-nineteen related deaths, which was 2% (https://world wide web.worldometers.info/coronavirus/ (accessed on 29 May 2021)). In the case of the Sputnik vaccine, one would thus have to vaccinate 22,000 people to forbid 1 death. In the case of the Moderna vaccine, one would take to vaccinate 3050 people to prevent 1 death. In the example of Comirnaty, the Pfizer vaccine, 6150 vaccinated people would prevent one death, although using the figure by Cunningham [8], information technology would be 12,300 vaccinations to forestall one death.
The side effects information reported in the Dutch register (world wide web.lareb.nl/coronameldingen (accessed on 27 May 2021)) are given in Table 3.
Thus, we need to accept that around sixteen cases volition develop severe adverse reactions from COVID-19 vaccines per 100,000 vaccinations delivered, and approximately 4 people will die from the consequences of beingness vaccinated per 100,000 vaccinations delivered. Adopting the point estimate of NNTV = 16,000 (95% CI, 9000–50,000) to preclude one COVID-19-related death, for every half-dozen (95% CI, ii–eleven) deaths prevented by vaccination, nosotros may incur iv deaths as a consequence of or associated with the vaccination. But put: As we prevent three deaths by vaccinating, we incur 2 deaths.
The risk–benefit ratio looks amend if we accept the stronger effect sizes from the phase three trials. Using Cunningham's guess of NNTV = 12,300, which stems from a not-peer reviewed comment, nosotros arrived at eight deaths prevented per 100,000 vaccinations and, in the all-time case, 33 deaths prevented past 100,000 vaccinations. Thus, in the optimum example, we risk four deaths to foreclose 33 deaths, a risk–do good ratio of 1:8. The risk–benefit ratio in terms of deaths prevented and deaths incurred thus ranges from 2:three to 1:8, although real-life information also support ratios as high as ii:1, i.e., twice every bit high a take a chance of death from the vaccination compared to COVID-nineteen, within the 95% confidence limit.
4. Discussion
The COVID-19 vaccines are immunologically effective and can—according to the publications—forestall infections, morbidity, and bloodshed associated with SARS-CoV2; however, they incur costs. Apart from the economic costs, there are insufficiently high rates of side furnishings and fatalities. The current figure is effectually iv fatalities per 100,000 vaccinations, equally documented past the most thorough European documentation system, the Dutch side furnishings register (lareb.nl). This tallies well with a recently conducted analysis of the U.S. vaccine agin reactions reporting system, which found 3.4 fatalities per 100,000 vaccinations, more often than not with the Comirnaty (Pfizer) and Moderna vaccines [12].
Is this a few or many? This is difficult to say, and the answer is dependent on one'south view of how severe the pandemic is and whether the common assumption that in that location is hardly any innate immunological defense force or cross-reactional immunity is true. Some argue that nosotros tin can assume cantankerous-reactivity of antibodies to conventional coronaviruses in 30–50% of the population [13,14,15,16]. This might explain why children and younger people are rarely afflicted by SARS-CoV2 [17,18,xix]. An innate immune reaction is difficult to gauge. Thus, depression seroprevalence figures [xx,21,22] may not only reflect a lack of herd amnesty, but also a mix of undetected cantankerous-reactivity of antibodies to other coronaviruses, as well as immigration of infection past innate immunity.
However, one should consider the simple legal fact that a expiry associated with a vaccination is different in kind and legal status from a expiry suffered equally a consequence of an incidental infection.
Our data should be viewed in the light of its inherent limitations:
The report which we used to gauge the NNTV was a single field written report, fifty-fifty though it is the largest to date. The other information stem from regulatory trials that were not designed to detect maximum effects. The field study was somewhat specific to the state of affairs in Israel, and studies in other countries and other populations or other post-marketing surveillance studies might reveal more benign clinical effect sizes when the prevalence of the infection is higher. This field written report likewise suffered from some bug, every bit a lot of cases were censored due to unknown reasons, presumably due to a loss to follow-upward. However, the regulatory studies compensate for some of the weaknesses, and thereby generate a somewhat more benign risk–benefit ratio.
The ADR database of the EMA collects reports of unlike kinds, by doctors, patients, and authorities. We observed (Figure one) that the reporting standards vary hugely across countries. Information technology might be necessary for the EMA and for national governments to install improve monitoring procedures in lodge to generate more reliable data. Some countries have tight reporting schemes, some written report in a rather loose mode. As nosotros have to assume that the boilerplate number of side effects is roughly similar beyond countries, we would expect a similar reporting quota. However, when inspecting the reports co-ordinate to countries, we can see a big variance. Our decision to use the Dutch data as a proxy for Europe was derived from this discovery. One might want to claiming this decision, merely we did not notice any data from other countries being more valid than those used here. Apart from this, our data tallied well with the data from the U.S. CDC vaccine agin reporting system [12], which indirectly validates our decision.
One might argue that it is always difficult to define causality in such reports. This is certainly truthful; yet, the Dutch data, peculiarly the fatal cases, were certified by medical specialists (https://www.lareb.nl/media/eacjg2eq/beleidsplan-2015-2019.pdf (accessed on 29 May 2021)), page xiii: "All reports received are checked for completeness and possible ambiguities. If necessary, additional information is requested from the reporting party and/or the treating doctor The report is entered into the database with all the necessary information. Side furnishings are coded according to the applicable (international) standards. Later on an individual assessment of the study is made. The reports are forwarded to the European database (Eudravigilance) and the database of the WHO Collaborating Centre for International Drug Monitoring in Uppsala. The registration holders are informed nigh the reports concerning their product.").
A recent experimental report showed that the SARS-CoV2 spike poly peptide is sufficient to produce endothelial damage [23]. This provides a potential causal rationale for the nearly serious and about frequent side effects, namely, vascular bug such equally thrombotic events. The vector-based COVID-19 vaccines tin produce soluble spike proteins, which multiply the potential damage sites [24]. The spike poly peptide also contains domains that may bind to cholinergic receptors, thereby compromising the cholinergic anti-inflammatory pathways, enhancing inflammatory processes [25]. A contempo review listed several other potential side effects of COVID-19 mRNA vaccines that may also emerge later than in the ascertainment periods covered here [26].
In the Israeli field study, the observation flow was half dozen weeks, and in the U.S. regulatory studies betwixt 4 to six weeks, a catamenia commonly causeless to be sufficient to see a clinical effect of a vaccine, because it would also be the time frame within which someone who was infected initially would fall sick and possibly dice. Had the observation menses been longer, the clinical event size might have increased, i.east., the NNTV could have become lower and, consequently, the ratio of benefit to damage could have increased in favor of the vaccines. Notwithstanding, equally noted to a higher place, at that place is also the possibility of side furnishings developing with some delay and influencing the chance–benefit ratio in the opposite management [26]. This should be studied more than systematically in a long-term observational study.
Some other bespeak to consider is that initially, mainly older persons and those at risk were entered into the national vaccination programs. Information technology is to be hoped that the tally of fatalities volition become lower every bit a upshot of the vaccinations, every bit the historic period of those vaccinated decreases.
Withal, we practice remember that, given the information, we should not expect to see whether more fatalities accumulate, but instead use the data available to study who might be at risk of suffering side furnishings and pursue a diligent route.
Finally, we note that from experience with reporting side effects from other drugs, merely a small fraction of side effects is reported to adverse events databases [27,28]. The median underreporting can be as high as 95% [29].
Given this fact and the high number of serious side effects already reported, the electric current political trend to vaccinate children who are at very low take chances of suffering from COVID-19 in the commencement identify must be reconsidered.
5. Conclusions
The nowadays assessment raises the question whether it would be necessary to rethink policies and use COVID-19 vaccines more sparingly and with some discretion only in those that are willing to accept the gamble because they feel more at hazard from the truthful infection than the mock infection. Peradventure information technology might exist necessary to dampen the enthusiasm by sober facts? In our view, the EMA and national authorities should instigate a safety review into the safety database of COVID-19 vaccines and governments should carefully consider their policies in calorie-free of these data. Ideally, independent scientists should behave out thorough case reviews of the very severe cases, so that there tin can exist evidence-based recommendations on who is likely to benefit from a SARS-CoV2 vaccination and who is in danger of suffering from side effects. Currently, our estimates prove that we have to accept four fatal and 16 serious side furnishings per 100,000 vaccinations in club to salvage the lives of 2–eleven individuals per 100,000 vaccinations, placing risks and benefits on the same social club of magnitude.
Writer Contributions
Conceptualization, H.Due west.; methodology, H.Due west.; writing—original typhoon, H.W.; guarantor, H.Westward.; checked the analysis for correctness and contributed to the writing. R.J.M.; analysis of the COVID-19 vaccination volumes reported by ECDC and the ICSR reports from EMA and graph production, W.A. All authors take read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This was a study on publicly available data and a secondary analysis, and equally such non subject to an ethical review.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the cited studies used in our analysis.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arvay, C.G. Genetische Impfstoffe gegen COVID-nineteen: Hoffnung oder Risiko. Schweiz. Ärztezeitung 2020, 101, 862–864. [Google Scholar]
- Ramasamy, G.N.; Minassian, A.One thousand.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-nineteen vaccine administered in a prime-boost regimen in immature and old adults (COV002): A single-blind, randomised, controlled, phase 2/iii trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Walsh, Eastward.E.; Frenck, R.Due west.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, Due south.; Neuzil, 1000.; Mulligan, K.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Condom and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. Northward. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.Grand.; Essink, B.; Kotloff, 1000.; Frey, Due south.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Prophylactic of the mRNA-1273 SARS-CoV-2 Vaccine. North. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Dagan, Due north.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, Yard.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-xix Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.Southward.; Kovyrshina, A.V.; Lubenets, Due north.L.; Grousova, D.M.; Erokhova, A.Due south.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim assay of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Cunningham, A.S. Rapid response: COVID-19 vaccine candidate is unimpressive: NNTV is effectually 256. BMJ 2020, 371, m4347. [Google Scholar]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, South.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, South.; Bittaye, Grand.; Clutterbuck, E.A.; et al. Safe and immunogenicity of the ChAdOx1 nCoV-nineteen vaccine confronting SARS-CoV-2: A preliminary written report of a phase 1/ii, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Klement, R.J.; Bandyopadhyay, P.S. The Epistemology of a Positive SARS-CoV-2 Test. Acta Biotheor. 2020. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Axfors, C.; Contopoulos-Ioannidis, D.One thousand. Population-level COVID-19 mortality risk for non-elderly individuals overall and for not-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020, 188, 109890. [Google Scholar] [CrossRef] [PubMed]
- Rose, J. A report on the U.South. vaccine agin events reporting organisation (VAERS) on the COVID-19 messenger ribonucleic acid (mRNA) biologicals. Sci. Public Health Policy Law 2021, 2, 59–80. [Google Scholar]
- Edridge, A.W.; Kaczorowska, J.M.; Hoste, A.C.; Bakker, K.; Klein, Yard.; Jebbink, M.F.; Matser, A.; Kinsella, C.; Rueda, P.; Prins, Yard.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Reed, C.; Lim, T.; Montgomery, J.M.; Klena, J.D.; Hall, A.J.; Fry, A.M.; Cannon, D.L.; Chiang, C.F.; Gibbons, A.; et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23–May 12, 2020. JAMA Intern. Med. 2020, 180, 1576–1586. [Google Scholar] [CrossRef]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, Due east.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Doshi, P. COVID-19: Do many people have pre-existing immunity? BMJ 2020, 370, m3563. [Google Scholar] [CrossRef]
- Lavine, J.S.; Bjornstad, O.N.; Antia, R. Immunological characteristics govern the transition of COVID-19 to endemicity. Scientific discipline 2021, 371, 741–745. [Google Scholar] [CrossRef]
- Brandal, Fifty.T.; Ofitserova, T.Due south.; Meijerink, H.; Rykkvin, R.; Lund, H.M.; Hungnes, O.; Greve-Isdahl, M.; Bragstad, K.; Nygård, K.; Winje, B.A. Minimal transmission of SARS-CoV-ii from paediatric COVID-nineteen cases in primary schools, Norway, August to November 2020. Eurosurveillance 2021, 26, 2002011. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Engerström, Fifty.; Nordenhäll, C.; Larsson, E. Open up Schools, COVID-19, and Child and Teacher Morbidity in Sweden. N. Engl. J. Med. 2021, 384, 669–671. [Google Scholar] [CrossRef]
- Lorent, D.; Nowak, R.; Roxo, C.; Lenartowicz, East.; Makarewicz, A.; Zaremba, B.; Nowak, Due south.; Kuszel, L.; Stefaniak, J.; Kierzek, R.; et al. Prevalence of Anti-SARS-CoV-2 Antibodies in Poznań, Poland, after the First Wave of the COVID-19 Pandemic. Vaccines 2021, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J. The infection fatality rate of COVID-19 inferred from seroprevalence data. Bull. World Health Organ. 2021, 99, 19F–33F. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, Eastward.; Mulaney, B.; Sood, N.; Shah, S.; Ling, E.; Bromley-Dulfano, R.; Lai, C.; Weissberg, Z.; Saavedra-Walker, R.; Tedrow, J.; et al. COVID-19 Antibody Seroprevalence in Santa Clara County, California. Int. J. Epidemiol. 2021, 50, 410–419. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon Cara, R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, 50.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Office via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Kowarz, Due east.; Krutzke, L.; Reis, J.; Bracharz, Due south.; Kochanek, Due south.; Marschalek, R. "Vaccine-Induced COVID-19 Mimicry" Syndrome: Splice reactions within the SARS-CoV-two Fasten open reading frame effect in Fasten poly peptide variants that may cause thromboembolic events in patients immunized with vector-based vaccines (non-peer reviewed preprint). Res. Sq. 2021. [Google Scholar] [CrossRef]
- Farsalinos, K.; Eliopoulos, Due east.; Leonidas, D.D.; Papadopoulos, G.E.; Tzartos, S.; Poulas, G. Nicotinic Cholinergic Organization and COVID-19: In Silico Identification of an Interaction between SARS-CoV-2 and Nicotinic Receptors with Potential Therapeutic Targeting Implications. Int. J. Mol. Sci. 2020, 21, 5807. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Well-nigh, Yard. Worse than the affliction? Reviewing some possible unintended consequences of the mRNA vaccines confronting COVID-nineteen. Int. J. Vaccine Theory Pract. Res. 2021, two, 38–79. [Google Scholar]
- Alatawi, Y.M.; Hansen, R.A. Empirical estimation of nether-reporting in the U.Due south. Nutrient and Drug Assistants Adverse Event Reporting Organization (FAERS). Expert Opin. Drug Saf. 2017, 16, 761–767. [Google Scholar] [CrossRef]
- Moore, T.J.; Bennett, C.50. Underreporting of Hemorrhagic and Thrombotic Complications of Pharmaceuticals to the U.S. Food and Drug Administration: Empirical Findings for Warfarin, Clopidogrel, Ticlopidine, and Thalidomide from the Southern Network on Agin Reactions (SONAR). Semin. Thromb. Hemost. 2012, 38, 905–907. [Google Scholar] [CrossRef]
- Hazell, L.; Shakri, S.A.Westward. Under-reporting of adverse drug reactions. A systematic review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef]
Figure 1. Individual safety case reports in association with COVID nineteen vaccines in Europe.
Figure ane. Individual safety case reports in association with COVID 19 vaccines in Europe.
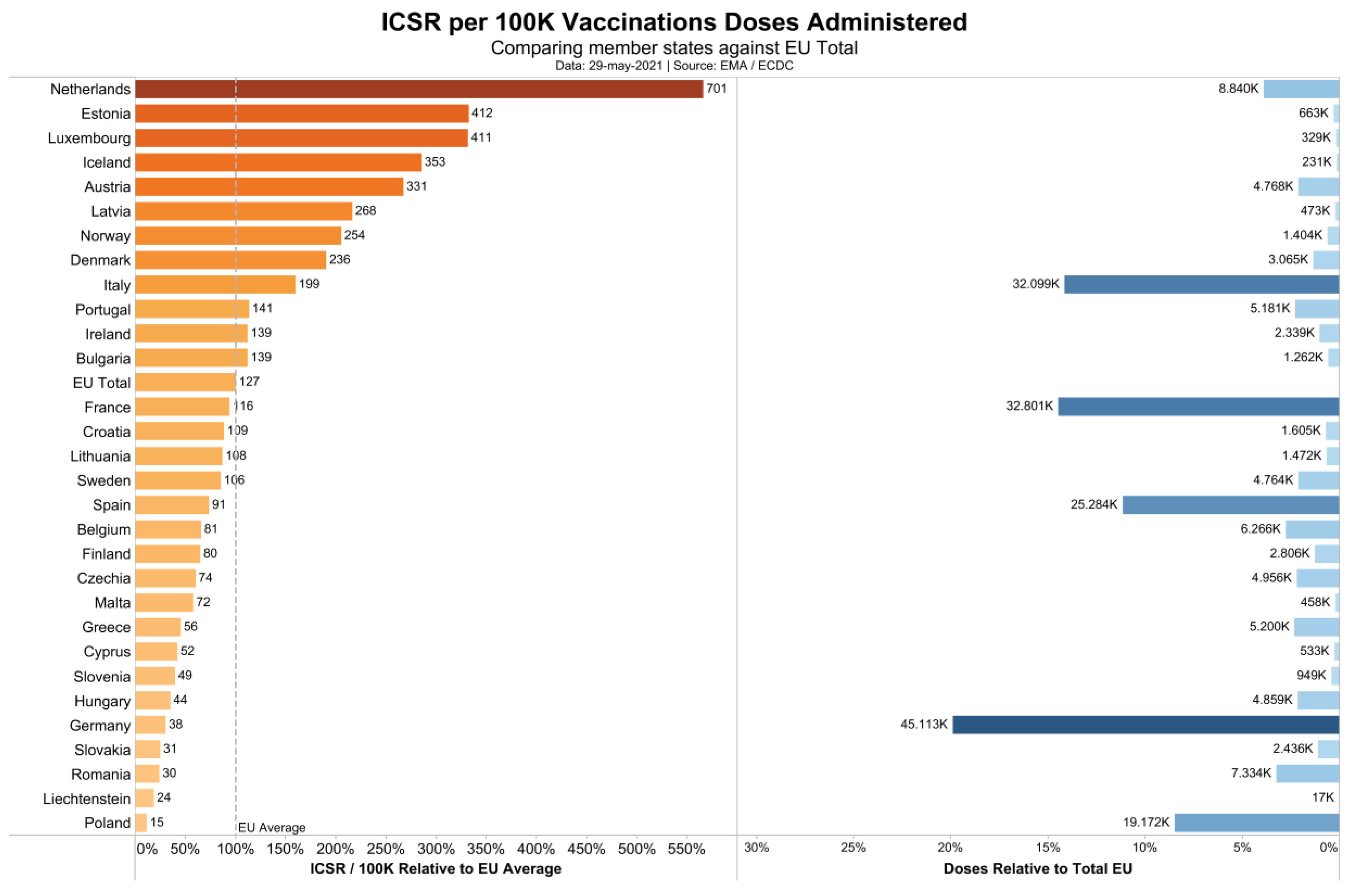
Table 1. Risk differences and number needed to vaccinate (NNTV) to foreclose one infection, ane case of symptomatic illness, and i death from COVID-nineteen. Information from Dagan et al. [6], Northward = 596,618 in each group.
Table 1. Gamble differences and number needed to vaccinate (NNTV) to prevent one infection, ane case of symptomatic illness, and one expiry from COVID-19. Data from Dagan et al. [6], N = 596,618 in each group.
| Documented Infection | Symptomatic Illness | Death from COVID-19 | ||||
|---|---|---|---|---|---|---|
| Period | Take chances Difference (No./one thousand Persons) (95% CI) | NNTV (95% CI) | Adventure Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) | Risk Difference (No./1000 Persons) (95% CI) | NNTV (95% CI) |
| 14–20 days after first dose | two.06 (1.lxx–2.forty) | 486 (417–589) | 1.54 (1.28–1.80) | 650 (556–782) | 0.03 (0.01–0.07) | 33,334 (fourteen,286–100,000) |
| 21–27 days later on offset dose | two.31 (1.96–2.69) | 433 (372–511) | 1.34 (1.09–1.62) | 747 (618–918) | 0.06 (0.02–0.xi) | 16,667 (9091–50,000) |
| 7 days after second dose to end of follow-up | 8.58 (half dozen.22–11.xviii) | 117 (90–161) | iv.61 (three.29–6.53) | 217 (154–304) | NA | NA |
Tabular array two. Number needed to vaccinate (NNTV) calculated from pivotal phase 3 regulatory trials of the SARS-CoV2 mRNA vaccines of Moderna, BioNTech/Pfizer, and Sputnik (the vector vaccine of Astra-Zeneca is not independent here, as the written report [ix] was active-controlled and not placebo-controlled).
Table 2. Number needed to vaccinate (NNTV) calculated from pivotal phase 3 regulatory trials of the SARS-CoV2 mRNA vaccines of Moderna, BioNTech/Pfizer, and Sputnik (the vector vaccine of Astra-Zeneca is non contained hither, as the report [ix] was active-controlled and non placebo-controlled).
| Vaccine | N Participants Vaccine Group | Due north Participants Placebo Grouping | CoV2 Positive Cease of Trial Vaccine Group | CoV2 Positive End of Trial Placebo Group | Absolute Risk Difference (ARD) | Number Needed to Vaccinate i/ARR |
|---|---|---|---|---|---|---|
| Moderna [5] $ | 15,181(14,550 *) | 15,170 (14,598 *) | xix (0.13%) one | 269 (1.77%) ane | 0.0165 | 61 |
| Comirnaty (BioNTech/Pfizer) [four] $ | 18,860 | xviii,846 | eight (0.042%) 2 | 162 (0.86%) two | 0.00817 | 123 |
| Sputnik Five [7] § | 14,964 | 4902 | thirteen (0.087%) **,3 | 47 (1%) **,iii | 0.0091 | 110 |
Table 3. Private example safety reports for the near widely distributed COVID-19 vaccines co-ordinate to the Dutch side effects register (www.lareb.nl/coronameldingen (accessed on 29 May 2021)), the absolute numbers per vaccine, and standardization per 100,000 vaccinations.
Table three. Private case safety reports for the most widely distributed COVID-xix vaccines according to the Dutch side effects annals (www.lareb.nl/coronameldingen (accessed on 29 May 2021)), the absolute numbers per vaccine, and standardization per 100,000 vaccinations.
| General Number of Reports (1) | Serious Side Furnishings (one) | Deaths (2) | Number of Vaccinations According to (3) | Number of Vaccinations According to ECDC (4) | |
|---|---|---|---|---|---|
| Comirnaty (Pfizer) | 21,321 | 864 | 280 | 5,946,031 | 6,004,808 |
| Moderna | 6390 | 114 | 35 | 531,449 | 540,862 |
| Vaxzevria (AstraZeneca) | 29,865 | 411 | 31 | i,837,407 | 1,852,996 |
| Janssen | 2596 | 7 | - | 142,069 | 143,525 |
| Unknown | 129 | fifteen | five | - | 540 |
| Full | sixty,301 | one.411 | 351 | 8,456,956 | 8,542,731 |
| Per 100,000 vaccinations according to Dutch data | 713.03 | xvi.68 | 4.15 | ||
| Per 100,000 vaccinations co-ordinate to ECDC | 705.87 | 16.52 | 4.11 |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This commodity is an open up access article distributed under the terms and conditions of the Artistic Commons Attribution (CC By) license (https://creativecommons.org/licenses/by/4.0/).
Source: https://www.mdpi.com/2076-393X/9/7/693/htm
0 Response to "Smith Family- Cosmetic Dentistry 1112 Nc H Ighway 120 Sneads Ferry N"
Post a Comment